
- Over 230,000 lawsuits have been filed against pharmaceutical companies in the U.S. in the past decade alone, underscoring the widespread issues patients face.
- More than 5,000 cases specifically relate to diabetes medications, including Ozempic, where patients have sought legal help to cover medical expenses or damages.
- Around 75% of those pursuing legal action report feeling that they were inadequately informed about potential risks before starting their treatments.
Healthcare lawsuits have become more common in recent years as people seek justice for complications linked to medical treatments. One example is the growing number of lawsuits involving Ozempic, a medication that doctors primarily prescribe to manage type 2 diabetes.
Ozempic has shown positive results for many, but some patients have reported unexpected side effects, leading them to question the drug’s safety and pursue legal action.
Understanding what drives these lawsuits, especially in healthcare, can shed light on how patients can seek justice.
People filing Ozempic lawsuits often claim that the doctors never fully informed them of the risks or that the drug led to severe, unanticipated health issues. Common side effects that have spurred these cases include nausea, vomiting, and potential long-term effects on the pancreas.
This lack of awareness about potential risks has raised concerns about the accountability of healthcare providers and drug manufacturers.
As lawsuits like this increase, patients continue to seek clarity and accountability in the healthcare system, striving for justice in an industry that directly affects their health and well-being.
What is Ozempic?
Ozempic is a known brand identity for a subcutaneous type of semaglutide injection. To reduce levels of blood sugar and the risks of cardiovascular diseases, Ozempic acts as an optimal solution.
Ozempic is primarily used for people who have type 2 diabetes. Ozempic is also one of the best solutions for managing weight.
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor that mimics what the GLP-1 hormone does. It is usually involved in regulating blood sugar.
Additionally, Ozempic is ideal for minimizing the symptoms of type 2 diabetes. It enhances insulin release and eventually reduces the amount of glucagon.
It is also known for reducing appetite and delaying gastric empties. However, Ozempic is not ideal for treating type 1 diabetes.
History of Ozempic
Ozempic was not initially designed to be an anti-obesity drug. When semaglutide came up, it was intended to be the ultimate therapy for diabetes. In 2017, Ozempic was finally publicized. It got its approval from the FDA and brought the effects of diabetes under control.
You might be wondering how it got its approval so fast. This is because Ozempic could control the glycemic levels in adults, whether it was a combination with other medications or for diabetes or something else.
Less than one year after Ozempic’s release in 2017, researchers discovered that this medication was also an optimal way to control obesity. Researchers understood the potential of weight management and made its market value higher.
It led to the development and approval of the FDA of a higher-dose formulation of semaglutide with the brand identity Wegovy for long-term weight management in adults with a minimum of one other condition related to weight, like high cholesterol or hypertension.
Ozempic soon became an ideal option for managing weight in the U.S. Later. Its popularity increased all around the world. However, the FDA got reports from FAERS about 10,000 adverse event reports regarding different variants of semaglutide.
After two years, despite several adverse event reports about this, it became the most common option for weight management. Later, the FDA included it in the list for drug shortages by 2023.
By this time, mainstream news outlets began to report the adverse effects of Ozempic. Users then shortly filed these lawsuits once the information got public.
Side Effects of Ozempic
Ozempic has many possible adverse effects, ranging from mild to severe. Knowing about these side effects is crucial for anyone considering the medication or is already using it. The following are the common and severe side effects:
- Dizziness
- Lack of appetite
- Sore throat
- Frequent tiredness or fatigue
- Constipation or diarrhea
- Upset stomach
- Headache
- Stomach pain
- Runny nose
Severe Side Effects
- Pancreatitis
- Kidney issues
- Gastroparesis
- Elevated heart rate
- Hypoglycemia (that is, low blood sugar level)
- Gallbladder disease
- Stomach flu
What is Gastroparesis?
Gastroparesis makes the stomach muscles not function adequately and empties the stomach. This condition is extremely weakening. This can even result in adverse effects of ongoing medical treatment. Even lawsuits have argued that Ozempic can worsen this condition as well.
Some of the common symptoms of gastroparesis include:
- The feeling of fullness following the commencement of a meal and for a long time after that
- Little to no appetite
- Gas and bloating, belching
- Upper abdominal pain
- Nausea and vomiting
- Heartburn
The symptoms listed above can spiral into other issues, such as diarrhea and nausea, thereby resulting in severe dehydration or malnutrition, especially if the patient tries to avoid eating due to their symptoms.
More complications, such as the presence of blood in your vomit or stool, indicate a severe problem that requires instant medical attention.
Is There a Lawsuit Against Ozempic?
Yes, there is! There’s an ongoing lawsuit against Ozempic, with over 1,090 personal injury cases filed as of October 2024, alleging severe gastrointestinal issues, vision loss, and other complications.
The lawsuits claim that Novo Nordisk, the manufacturer, failed to adequately warn consumers about potential risks associated with Ozempic.
Common Allegations:
- Gastroparesis: a condition causing food to move slowly through the stomach, leading to nausea, vomiting, abdominal pain, and malnutrition/
- Vision Loss: reports of blindness and nonarteritic anterior ischemic optic neuropathy (NAION) linked to semaglutide, the active ingredient in Ozempic.
- Intestinal Blockage/Ileus: severe digestive issues, including intestinal obstruction and persistent vomiting.
Lawsuit Timeline:
- August 2023: The first Ozempic lawsuit was filed, with plaintiffs seeking compensation for medical expenses, lost income, and quality of life damages.
- September 2023: The FDA added intestinal blockage and ileus warnings to Ozempic’s label.
- July 2024: Lawyers began investigating potential vision loss lawsuits related to Ozempic.
If you’ve experienced severe side effects after taking Ozempic, it’s essential to consult with a lawyer to discuss potential compensation. But what do you need to do about it? How to go about it? And is there anything else that you might need to know about this class action lawsuit?
Ozempic Lawsuit: A Nerve-Wracking Product Liability Case!
The Ozempic lawsuit is about patients who have experienced unexpected and sometimes serious side effects from using Ozempic, a drug mainly prescribed to help people with type 2 diabetes manage blood sugar levels.
Ozempic, produced by Novo Nordisk, has shown effectiveness in controlling diabetes and even aiding in weight loss, which led to its growing popularity.
However, as more people started using it, reports of severe side effects began surfacing, raising concerns about the drug’s safety.
At the core of these lawsuits, patients claim that the doctors did not adequately warn them about the potential risks involved with Ozempic.
People have reported common side effects, like nausea, vomiting, and diarrhea. However, some patients allege that they experienced more severe complications, including pancreatic issues and thyroid cancer.
These claims have led to a wave of legal actions as affected individuals seek compensation for the impact on their health and quality of life.
A significant issue highlighted in the lawsuits is the lack of transparency regarding Ozempic’s side effects.
Many patients feel that Novo Nordisk and their healthcare providers did not fully inform them about the potential risks before taking the medication.
This has raised broader questions about patient rights and the responsibility of pharmaceutical companies to disclose all risks associated with their products.
Ozempic Lawsuit Timeline
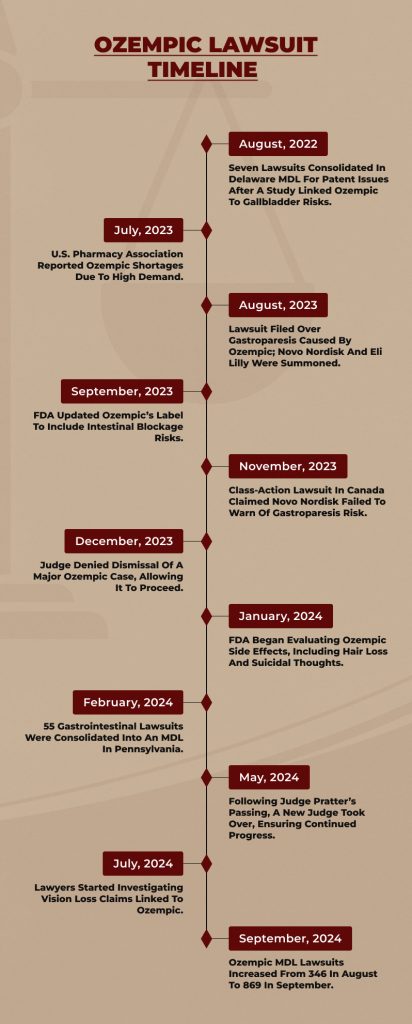
To fully understand how the Ozempic Lawsuit has been gaining momentum, you must take a look at the timeline of the case:
September 2024
The Ozempic lawsuit numbers are quickly rising. At the beginning of August, there were 346 active lawsuits in the MDL (multidistrict litigation). By early September, that number had jumped to 869.
July 2024
Lawyers are looking into claims of vision loss and blindness related to semaglutide, the active ingredient in Ozempic. A study published in JAMA Ophthalmology in 2024 found a link between semaglutide and a rare eye condition called nonarteritic anterior ischemic optic neuropathy (NAION).
June 2024
On June 6th, the Judicial Panel on Multidistrict Litigation reassigned the Ozempic MDL to Judge Karen S. Marston. She held a status meeting with attorneys soon after. By June 11th, there were no new official orders. Still, it’s likely Judge Marston will carry on the work of the late Judge Pratter by helping attorneys choose bellwether cases and coordinate discovery.
May 2024
The unexpected passing of Judge Gene E. K. Pratter on May 17th left clients uncertain about case delays in the MDL. However, lawyers assured clients they are dedicated to moving the litigation forward and keeping progress on track.
In May, Judge Pratter appointed Paul Pennock from Morgan & Morgan and other attorneys to the Plaintiff’s Committee, ensuring experienced case oversight.
March 2024
Litigation was still new as defendant attorneys from Novo Nordisk and Eli Lilly filed appearances, and more plaintiffs continued to join the MDL.
February 2024
At least 55 lawsuits related to gastrointestinal issues caused by Ozempic and similar drugs were combined into a multidistrict case in the Eastern District of Pennsylvania. Lawyers expect this number to eventually reach 10,000.
January 2024
The FDA began evaluating reports of side effects from Ozempic, including hair loss, suicidal thoughts, and aspiration under anesthesia, following several reports in their Adverse Event Reporting System.
December 2023
Judge James Cain Jr. denied Novo Nordisk’s attempt to dismiss Jaclyn Bjorklund’s case but allowed all other claims to proceed. Plaintiff lawyers filed a motion to consolidate Ozempic cases in Louisiana District Court.
November 2023
The users filed a class-action lawsuit in British Columbia, Canada, accusing Novo Nordisk of failing to warn about potential complications, particularly gastroparesis. This case reflects similar lawsuits in other regions.
September 2023
The FDA updated Ozempic’s warning label to include intestinal blockage, obstruction, and ileus risks.
August 2023
Jaclyn Bjorklund filed a lawsuit for gastroparesis caused by Ozempic and Mounjaro. The court summoned Novo Nordisk and Eli Lilly on August 3rd, and Novo Nordisk later filed a motion to dismiss her case in November.
July 2023
The ASHP, the largest U.S. pharmacy association, announced that Novo Nordisk faced shortages of Ozempic and Wegovy due to high demand.
August 2022
Seven Novo Nordisk lawsuits were consolidated in Delaware MDL for patent issues as generic makers applied for FDA approval to make Ozempic versions. This followed a JAMA study linking Ozempic to gallbladder risks, prompting new lawsuits.
The Newest November 7th Ozempic Lawsuit Update
- New Warning for Pulmonary Aspiration: November 7th, 2024
- Focus on Gastric Emptying Studies: November 4th, 2024
- Slower MDL Growth: November 1st, 2024
On November 1st, we saw the total number of cases coming up to 1,221. This was After a surge of over 400 cases in August and September and 131 in October.
On November 4th, a key question emerged: can plaintiffs prove that Ozempic causes gastroparesis, and how reliable are diagnoses?
This uncertainty led to a stronger focus on gastric emptying studies, measuring how quickly food leaves the stomach and being the most accurate way to diagnose gastroparesis.
These studies will likely become essential evidence in proving that GLP-1 drugs impact stomach motility. With this objective data, plaintiffs can support their claims more scientifically and separate true cases of gastroparesis from other digestive issues.
On November 7th, federal regulators issued a new warning about the risk of pulmonary aspiration with popular GLP-1 drugs, such as Ozempic, Wegovy, and Mounjaro. This warning links these medications to a risk of serious complications during elective surgeries.
Because GLP-1 drugs slow stomach emptying, patients using these medications may experience vomiting and dangerous aspiration when under anesthesia. While experts had flagged this concern earlier in the year, the official warning was only added now.
The FDA advises all patients taking GLP-1 medications to inform their healthcare providers before surgical procedures.
This new warning highlights a broader trend: while people still use these medications widely, they do to completely understand the effects. Each warning update reminds us that there’s more to learn about both the risks and benefits of Ozempic and similar drugs.
Ozempic Lawsuit: Settlement Amount, Filing Process, and Eligibility
Ozempic settlement payment estimates vary greatly, typically from $70,000 to $500,000.
People generally estimate the potential settlements for claims involving serious injuries to be between $350,000 and over $1 million. We can expect settlements in situations involving wrongful death or severe gastroparesis to range from $400,000 to $700,000.
Depending on several factors, like the extent of injuries and associated medical issues, settlement amounts for Ozempic claims might vary significantly.
Due to the greater impact on the plaintiff’s life, settlements for claims involving catastrophic injuries typically include a higher range.
How to File an Ozempic Lawsuit?
Due to the complexity of filing an Ozempic claim, legal counsel is essential. The procedure entails speaking with an attorney to determine eligibility and obtain the required documentation.
Because of its reputation as a plaintiff-friendly court, the Pennsylvania federal court—particularly the Eastern District—is a major forum for Ozempic cases. Most states have a two-year statute of limitations for bringing a case after taking Ozempic.
To prove their eligibility and initiate an Olympic case, plaintiffs may have to submit a Plaintiff Fact Sheet and provide a doctor’s diagnosis of ailments such as gastroparesis.
The choice of an experienced legal team in drug litigation greatly impacts the result of an Ozempic lawsuit. The particular requirements for bringing a lawsuit and the required paperwork are described in the following subsections.
Eligibility: Who Can File an Ozempic Lawsuit?
Generally speaking, patients who were 75 years of age or older when they began using the medication are not qualified to file.
One must demonstrate that taking Ozempic caused serious health problems to be eligible for a lawsuit.
Candidates for Ozempic lawsuits are required to submit documentation of their use, including prescription drugs and medical documents.
People who develop intestinal blockage or severe gastroparesis soon after taking Ozempic might have a strong case in the Ozempic class action lawsuit.
Justice In Healthcare
The Ozempic lawsuit emphasizes the importance of accountability in healthcare. Patients want assurance that they can trust the medications prescribed to them and expect transparency about possible side effects.
For those affected, this lawsuit is a way to seek justice, compensation for medical expenses, and even changes in how drug information is communicated to the public, helping protect future patients from similar experiences.
Read Also:
- Difference Between Negligence And Malpractice?
- The Price of Negligence: Nevada Medical Malpractice Lawsuits
- 8 Fascinating Aspects of Medical Testimony that will Surprise You!











0 Reply
No comments yet.