
Today’s topic: Power Stick Deodorant Recall FDA!
The power stick deodorant recall FDA that has been instigated, has raised concerns among consumers of this affordable grooming aid.
A safety analysis carried out on these deodorant sprays showed that they contained the carcinogen benzene.
This increases the danger of leukemia in users. It is, therefore, vital that the company is closely monitored as it seeks to recall the products from the market. Various class-action suits are currently underway in court as of early 2026.
These suits claim that the company was aware of the dangers of exposure to these chemicals during usage. Consumers of this product must inspect the contents of their bathroom cabinets.
In this article, we will elaborate on the following:
- The specific reasons behind the safety alert and benzene contamination.
- Which batch numbers and scents are part of the active recall.
- Steps for identifying affected products and securing a full refund.
- Health symptoms to watch for after using contaminated aerosol sprays.
Understanding The Root Cause Of The Recall
The Power Stick Deodorant Recall FDA began when independent testing labs reported high levels of benzene in a number of aerosol products.
Upon this report, there was an immediate investigation into the propellant system used by this brand of deodorant.
“The Food and Drug Administration has issued a recall on 67,000 cases of Power Stick antiperspirant.” – NBC News

The Problem With Aerosol Propellants
Butane or propane is normally used in aerosol sprays to force the liquid out of the can. These raw materials, when not refined properly, sometimes carry industrial impurities like benzene. It is this sort of contamination that has caused the power stick deodorant recall FDA.
How Benzene Enters The Product
Benzene is not an intentionally added ingredient in deodorant; rather, it was introduced into the supply chain during the manufacturing of the gas that makes the spray work.
Therefore, the source of these gases is highly focused on within the power stick deodorant FDA recall.
FDA Classification Of The Hazard
The agency characterizes this as a Class II recall. This means the product may cause temporary health problems, but the possibility of serious permanent damage exists for long-term users.
Due to this fact, the power stick deodorant recall FDA is becoming one of the high priorities for federal inspectors.
Affected Products And Batch Identification
Not all products under the brand name are included in the power stick deodorant recall by the FDA.
Only the specific aerosol sprays that were manufactured during a particular period are the ones that are dangerous to the public.

Specific Scents Named In The Alert
The ‘Power Stick Deodorant Recall FDA’ mentions “Power Stick Intensity” and “Power Stick Sport.” If the scents match these, then it is necessary to verify the dates to avoid exposure to chemical substances.
Where To Find The Batch Code
The lot number is most likely printed on the bottom of the aluminum container in black ink. This is an important piece of information to use in checking whether your unit is part of the Power Stick deodorant recall FDA.
Why Non-Aerosols Are Excluded
Solid sticks and gel applicators do not have any form of chemical propellant. Since the benzene problem is associated with the spray mechanism, other forms, such as solid sticks, are not included in the power stick deodorant recall FDA regulation. You can carry on with solid sticks without any concerns.
Health Risks Associated With Benzene Exposure
The key concern when considering the power stick deodorant recall FDA is the administration of inhaled toxins into the human system.
It should be noted that benzene is a human carcinogen, which impacts the blood and bone marrow over years of exposure.
The Connection Between Benzene And Leukemia
Medical research has proven that exposure to benzene over an extended period of time leads to blood cancers.
Although the product would not cause cancer from one-time use, the power stick deodorant recall by the FDA seeks to eliminate its continuous exposure over several months.
Short-Term Physical Symptoms
Apparently, there were complaints of headaches or skin irritations after using these products. If you are feeling light-headed after applying your product, fresh air is recommended as per the Power Stick deodorant recall FDA.
Vulnerable Populations And Risk Factors
Children and teenagers tend to use a greater amount of spray than adults in small, unventilated bathrooms. Not only does this increase the dosage of benzene breathed in, but the power stick deodorant recall FDA significance becomes increasingly relevant for parents with kids.
How To Get Your Refund & Return Products
The manufacturer is offering to refund the money to those who have been affected by the power stick deodorant recall FDA. It does not matter whether you have the receipt or not, as long as you have the photo of the product.
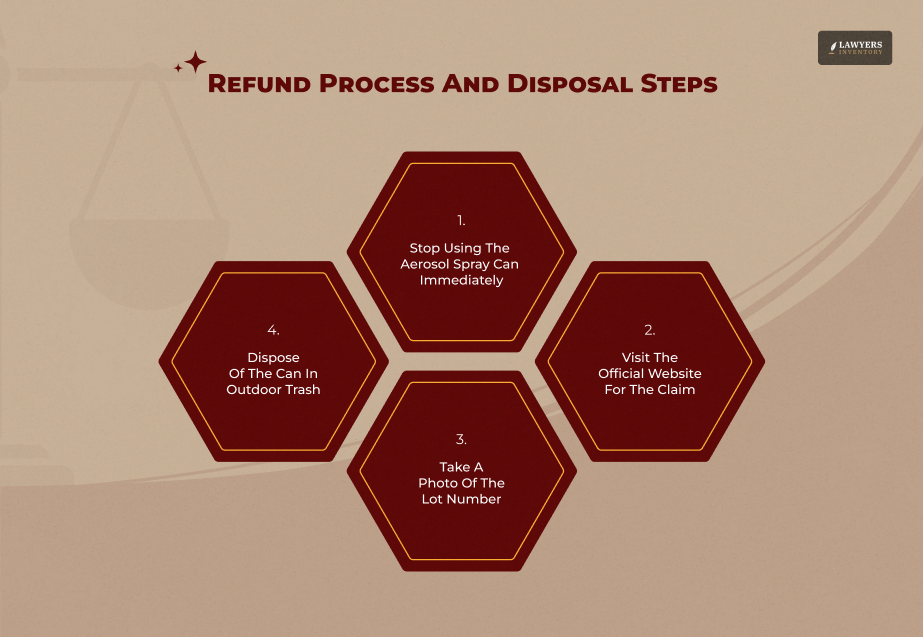
Submitting Your Online Claim
In order to start the process, you will need to visit the brand’s specific recall website. In order to qualify for the power stick deodorant recall FDA reimbursement, you will need to enter the batch code located on your can.
What To Do Without A Receipt
Most retailers also realize that no one reasonably expects to keep receipts for cheap grooming products. As a result of the power stick deodorant recall FDA, retailers have begun to accept the product for exchange or store credit [USA Today].
Safe Disposal Procedures
Do not dispose of the can into the air or drain. As the contents are pressurized and contaminated, it is best to dispose of the power stick deodorant recall in accordance with FDA regulations by simply putting it into the waste bin, but away from your living spaces.
Legal Claims And Consumer Rights
The FDA power stick deodorant recall has given way to a number of major filings against its parent company.
Attorneys note the company should have performed more rigorous tests for impurities prior to shipping into discount stores.

Joining Class Action Suits
You may have more than just a simple refund if you have been using them long enough. The FDA power stick deodorant recall has created a way for users to seek compensation for possible health risks.
Proof Of Product Consumption
Save your empty cans, or take clear photos of the labels before you throw them away. This will be great evidence if you decide to take part in any legal actions about power stick deodorant recall, FDA.
Corporate Responsibility In 2026
Under new legislation, beginning in 2026, companies will have to be transparent about who supplies them.
The FDA recall of Power Stick deodorant is a wake-up call for other brands. It will inspire them to go ahead and conduct audits of their propellant sources in order to avoid similar disasters.
Expert Tips For Safe Grooming
The power stick deodorant recall FDA emphasizes the importance of delivering the message to consumers concerning the information available regarding the products they apply to their bodies. By changing their behavior, they can limit their exposure to hidden industrial chemicals.
Switching To Non-Aerosol Altern
The very best way to avoid the risks that were observed by the power stick deodorant recallFDA is by totally avoiding the use of aerosols. Stick deodorants, creams, and roll-ons are not associated with the risk of benzene contamination.
Enhancing Bathroom Ventilation
If you must use a spray, you should make sure that your exhaust fan is switched on or a window open, as this disperses any dangerous gases that may be present, even though there is no active power stick deodorant recall FDA for your specific brand.
Monitoring Future Recall Notices
Safety alerts may occur at any time. By checking the government databases, you can stay ahead of problems like the power stick deodorant recall and prevent your loved ones from getting harmed.
Frequently Asked Questions (FAQs):
There has been much confusion and uncertainty about the power stick deodorant recall by the FDA among many consumers, and how it impacts their lives. We have answered some of the basic and frequently asked questions about the power stick deodorant recall by the FDA.
Yes, you can continue to use these items. The power stick deodorant recall by the FDA only involves the spray cans that use specific gas propellants. Liquid soap and shampoo do not contain these gases and have negative tests for benzene content.
Do not panic, as it is unlikely to cause an immediate health crisis, but wash the area with soap and water and ensure your area is well ventilated. However, it is recommended that you stop using the can and follow the Power Stick Deodorant Recall FDA instructions regarding the refund.
While this is happening in the US, which is under the jurisdiction of the FDA, there has been a recurrence of this for Canadian and European consumers. If you are in any of these countries, visit your regional health department website to find out about this power stick deodorant recall FDA.
In case you have recently used these products, we recommend talking to a medical expert to hear your concerns about chemicals.
Read Also:
- Turkeyfoot Creek Creamery Ice Cream Recall – What’s Happening
- The Complete Guide To Miracle Moo Bovine Colostrum Benefits
- Herbalife Relaxation Tea Recall: Safety Risks And Future Course












0 Reply
No comments yet.