
The Suboxone class action lawsuit has become one of the largest product liability cases in history, with nearly 670 cases filed.
This sheer number of lawsuits has caught attention due to the widespread use of Suboxone, a medication originally marketed to treat opioid addiction.
The lawsuits highlight claims that the manufacturers of Suboxone engaged in misleading marketing practices. Moreover, they put patients at risk without fully informing them about the drug’s potential side effects and risks.
Patients and families allege that the doctors did not warn them adequately about the potential for dependency and other adverse effects.
In this article, I will discuss the Suboxone lawsuits, examining the legal, medical, and consumer impacts. Besides, understanding these implications is important for those directly affected and anyone interested in pharmaceutical transparency and patient safety.
With so many lives impacted, these cases offer insights into how pharmaceutical companies must responsibly inform and protect their consumers.
This case is significant because it raises broader questions about pharmaceutical practices and product liability.
The legal case surrounding Suboxone, similar to the Ozempic lawsuit, offers a key example of the importance of patient rights, informed consent, and ethical standards in the healthcare industry.
What is Suboxone and How Does It Work?

Suboxone is a medication that doctors primarily prescribe in the treatment of opioid addiction. It’s a combination drug, meaning it combines two ingredients: naloxone and buprenorphine.
Naloxone is an opioid antagonist. Its role is to prevent misuse by blocking opioid effects if Suboxone is injected instead of taken as prescribed. This is usually done as a dissolvable film under the tongue.
Buprenorphine, on the other hand, is a partial opioid agonist. It helps to ease withdrawal symptoms and reduce cravings by mimicking some effects of opioids without producing the same “high.”
The makers of Suboxone marketed it as a safer alternative to traditional addiction treatments. For instance, these include medicines like methadone, promising fewer side effects and a lower risk of dependency.
The idea was to provide patients struggling with addiction with a pathway to recovery while minimizing the likelihood of misuse or overdose. Doctors have widely prescribed the drug for many patients. Additionally, it has proved itself to be beneficial in managing opioid addiction effectively.
However, concerns about Suboxone’s safety profile have led to hundreds of lawsuits. Patients have reported dependency issues, with some alleging that the drug itself is highly addictive.
Other reported side effects include nausea, headaches, withdrawal symptoms, and, in rare cases, respiratory problems. These side effects, along with accusations that the makers had not fully disclosed the drug’s risks, have fueled the ongoing legal battles around Suboxone.
Overview of the Suboxone Class Action Lawsuit
The Suboxone lawsuits have gained attention because of the sheer scale and nature of the claims. Yes, there are over 600 cases filed against the drug’s manufacturer, Indivior.
The lawsuits are primarily class actions, uniting patients who allege harm or deception related to Suboxone’s use and marketing. Each case sheds light on the broader allegations surrounding the drug’s marketing practices, addiction risks, and the company’s conduct.
History of the Suboxone Class Action Lawsuit
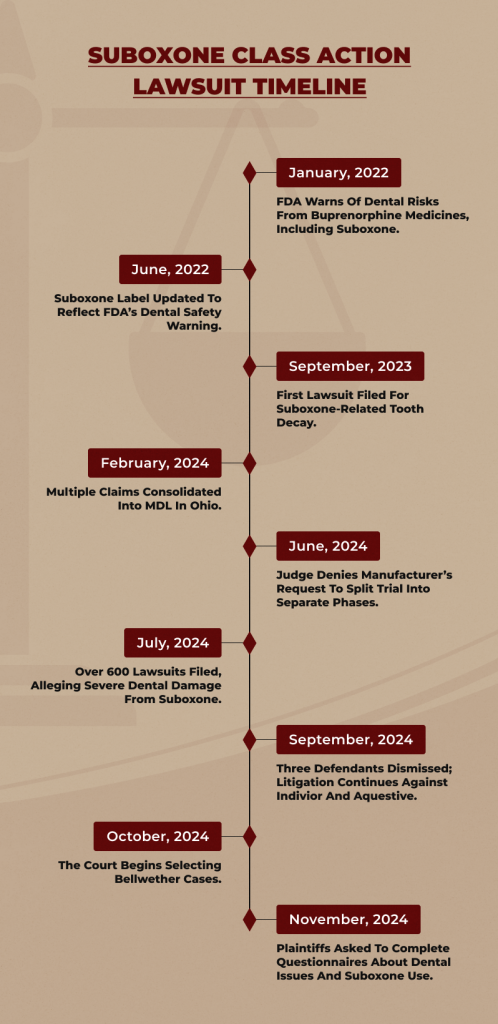
On September 23, 2023, the Northern District of Ohio received the first lawsuit. People have filed more than 100 lawsuits alleging Suboxone caused harm in federal courts across several jurisdictions as of November 2023.
The Northern District of Ohio is where most of the cases are located. To date, no cases claiming that Suboxone treatment caused oral damage have been settled.
In January 2024, the Joint Panel on Multidistrict Litigation convened to deliberate on consolidating all Suboxone litigation filed in federal courts.
A combined multidistrict lawsuit (MDL) could speed up the case resolution process. No order, including a decision about MDL consolidation, has yet been issued.
The FTC has previously brought a class action antitrust case against Indivior and RB Group.
According to that case, the defendants’ deliberate actions to block competition from less expensive generic medications drove consumers to pay more for Suboxone.
In 2019, RB Group agreed to pay $50 million to consumers as part of a settlement with the FTC. In 2020, Indivior also settled and consented to reimburse customers $10 million.
Indivior settled with several states in June 2023 and then paid $30 million in August to settle a class action lawsuit filed by health plans. Indivior paid $385 million to 70 drug distributors in November 2023 as part of a settlement of an antitrust lawsuit.
In addition, the court charged Indivior and RB Group with criminal and civil offenses for allegedly using misleading marketing techniques. Several states and the US Department of Justice (DOJ) filed charges.
The allegations against Indivior and RB Group were settled separately in 2019. To resolve DOJ and state allegations, Indivior agreed to pay $600 million, and RB Group agreed to pay $2.1 billion.
Summary of Claims
The main allegations in these lawsuits include improper marketing, failure to warn, and misleading claims about Suboxone’s addiction potential.
Many plaintiffs argue that Indivior marketed Suboxone as a safe and less addictive treatment for opioid dependence but allegedly minimized or omitted risks associated with long-term use.
Some patients claim that they became dependent on Suboxone, suffering withdrawal symptoms when trying to stop, which was contrary to their expectations.
Others allege that Indivior misled consumers by emphasizing Suboxone’s effectiveness without adequately explaining its potential for dependency.
Class Action Details
The class action lawsuits involve a large, diverse group of plaintiffs from various regions, reflecting the drug’s widespread impact.
People have filed cases across the United States, from Pennsylvania to Kentucky, with plaintiffs including individuals of different ages and backgrounds.
All of them were seeking help for opioid addiction. However, they ended up facing unexpected challenges with Suboxone.
The lawsuits collectively allege that the makers did not communicate the drug’s risks. Additionally, they also said that the company misled the patients into believing Suboxone was a low-risk treatment option.
Defendant’s Response
Indivior, the drug’s manufacturer, has consistently denied the allegations. They have asserted that Suboxone is effective and safe when people use it as per the doctor’s direction.
The company argues they had outlined the drug’s risks in its labeling. Additionally, they also mentioned that addiction potential is well-known in addiction treatment medications.
Indivior has settled some cases in the past, but it continues to contest many ongoing lawsuits, maintaining that it adhered to safety and marketing regulations.
Understanding the Legal Concepts Related to Class Action Lawsuit for Suboxone
In product liability cases like the Suboxone lawsuits, several key legal concepts play a major role in the claims and proceedings.
But why is it important? These legal concepts—product liability, failure to warn, and misleading marketing—highlight the importance of transparency and accountability in the pharmaceutical industry, where patient safety relies on access to accurate information.
Therefore, understanding these terms helps make sense of the legal grounds on which patients are challenging the drug’s manufacturer, Indivior, over alleged harm from the drug.
What is Product Liability
Product liability refers to the legal responsibility that manufacturers, suppliers, or sellers hold for producing or selling a defective product that causes harm to consumers.
In the Suboxone lawsuits, plaintiffs argue that Indivior failed to deliver a safe product or adequately communicate its risks.
Product liability claims typically involve design defects, manufacturing errors, or inadequate warnings.
In this case, the primary focus is on alleged failure to warn and misleading marketing—two issues closely linked to product liability in the pharmaceutical industry.
Failure to Warn
The “failure to warn” claim is at the heart of many Suboxone lawsuits. In these cases, plaintiffs allege that Indivior did not provide sufficient warnings about Suboxone’s potential for addiction and withdrawal symptoms.
The idea is that patients have a right to clear and honest information about all risks associated with a medication to make informed choices.
Many patients claim that the doctors or the packaging of the medicine did not fully inform them of Suboxone’s potential for dependency. Additionally, they mentioned that it put them at risk of unintended long-term dependence.
Misleading Marketing Claims
Misleading marketing is another significant allegation in these lawsuits. Patients accuse Indivior of promoting Suboxone as a low-risk, highly effective solution for opioid addiction without adequately disclosing its potential downsides.
In legal terms, misleading marketing claims occur when a company’s advertisements or statements create false impressions about a product’s benefits, risks, or uses.
In the Suboxone case, patients argue that the marketing strategies downplayed the risk of dependency and withdrawal, potentially leading to serious health consequences.
Timeline of the Suboxone Class Action Lawsuit
Filing for Suboxone Class Action Lawsuit Compensation
Indivior and RB Group have faced legal challenges over Suboxone, involving civil and criminal charges for misleading marketing and antitrust violations. They have resolved those issues with final settlement payments in 2023.
However, new lawsuits are surfacing, with claims that Suboxone can cause severe dental damage—a risk patients say they weren’t warned about.
Common dental issues linked to Suboxone use include tooth decay, oral infections, cavities, cracked teeth, root canals, tooth loss, and injuries to gums and tongue.
If you’re considering a Suboxone lawsuit, it’s best to consult a personal injury lawyer, especially one experienced in drug-related cases. A lawyer can help evaluate your case and navigate any deadlines, as some claims may be affected by statutes of limitations.
With Suboxone cases potentially moving toward a Multi-District Litigation (MDL), working with a lawyer familiar with mass torts could be beneficial.
To build a strong case, you’ll need to gather evidence. This includes medical records showing your Suboxone use, dental records documenting damage, and opinions from doctors or medical experts.
Although prior settlements were related to deceptive marketing, no settlements for dental damage claims have been reached, so compensation amounts are currently unknown.
Patient’s Safety and Rights in Medication Use
Ensuring patient safety and rights is essential, especially regarding medications like Suboxone, which carry specific risks.
The large number of Suboxone-related lawsuits highlights the importance of patient awareness. It could drive changes in how medications are prescribed and regulated.
Patient Awareness and Consent
One of the core aspects of patient rights is informed consent, which means patients must have access to clear, comprehensive information about any medication’s potential benefits and risks before making a decision.
For Suboxone, which combines buprenorphine and naloxone to treat opioid addiction, patients must understand its risks, such as dependency and withdrawal symptoms.
Without fully understanding these aspects, patients may unknowingly face health challenges. Proper informed consent requires doctors and manufacturers to be transparent about such risks, empowering patients to make safer, more informed decisions.
Regulatory Implications
The wave of lawsuits against Suboxone’s manufacturers may influence regulatory changes to improve patient safety.
For example, the FDA might consider stricter guidelines for labeling, especially for medications with a high risk of dependency. Clearer labeling and stronger regulation of marketing practices could help prevent future harm.
Additionally, there may be an emphasis on improving how potential side effects are communicated to healthcare providers and patients, ensuring a more proactive approach to safety.
Advice for Patients
Patients can take steps to protect themselves when using any medication. Asking detailed questions about prescribed drugs, including possible side effects and alternatives, can provide clarity and peace of mind.
Independent research is also helpful, as reliable sources can offer insights into a medication’s full profile. Patients who encounter unexpected side effects should report these to their doctor and regulatory bodies like the FDA.
This proactive approach is an important part of maintaining personal safety and promoting better standards in healthcare.
The Suboxone Class Action Lawsuit Might Impact the Future of Pharmaceutical Marketing
The extensive class action lawsuits surrounding Suboxone could lead to major changes in how pharmaceutical companies approach marketing.
With over 600 lawsuits alleging that Suboxone’s manufacturer, Indivior, used misleading marketing tactics, this case has caught the attention of both consumers and regulators.
Moreover, it has set an example for how society can handle deceptive advertising practices in the future.
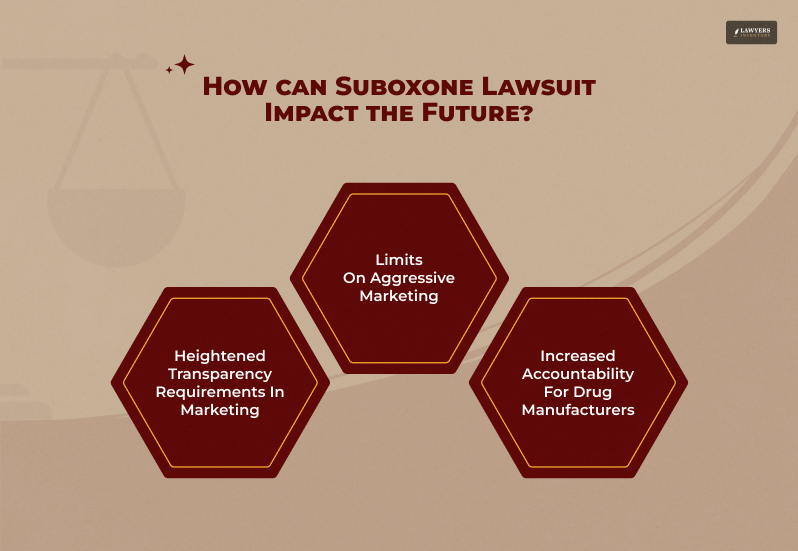
Heightened Transparency Requirements
One potential outcome of the Suboxone lawsuits is increased transparency in pharmaceutical marketing. Patients who used Suboxone have claimed they did not know about the risks, such as dependency and withdrawal. These were associated with the drug.
Moving forward, the FDA and other regulatory bodies may push for stricter guidelines on how drug risks are communicated, requiring companies to highlight any dependency risks or side effects.
This shift would ensure patients and healthcare providers receive a more accurate picture, helping them make informed decisions.
Limits on Aggressive Marketing
The Suboxone case also calls into question aggressive marketing tactics often used in pharmaceuticals.
Lawsuits allege that Indivior promoted Suboxone as safer and more effective than it was, influencing patients and doctors alike.
This case could drive regulatory bodies to curb overly persuasive advertising. This is true especially when it involves life-impacting drugs like those used to treat addiction.
Additionally, stricter advertising guidelines could reduce the likelihood of patients receiving misleading information, ultimately protecting their health.
Increased Accountability for Drug Manufacturers
Finally, this lawsuit emphasizes the need for drug manufacturers to be accountable for the claims they make.
If the case concludes in favor of the plaintiffs, it might lead to tighter restrictions on how drugs are marketed. Furthermore, there can be heavier penalties for companies that mislead consumers.
For patients, this change would mean greater assurance that the information they receive from pharmaceutical companies is accurate and prioritized for their well-being.
Read Also:
- Paul Mackoul MD Lawsuit: The Case That Taught Us About Patient’s Rights and Accountability in Healthcare
- Smoothstack Lawsuit: Beware of Unlawful Wage Scheme and Employment Contracts
- Kennedy Funding Lawsuit and the Exploitation of Arkansas Statute of Frauds











0 Reply
No comments yet.